A BRIGHT SOLUTION
FOR DRY EYES

DRY EYE MEDICAL DEVICE
Lumenis, the inventor of IPL, presents the first and only IPL FDA-approved for the management of dry eye disease due to MGD.
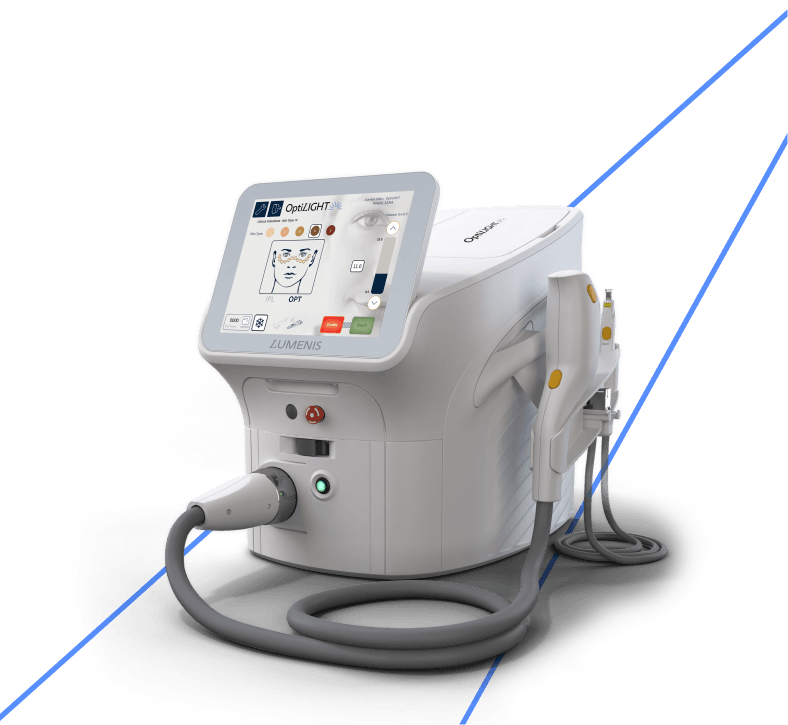
Introducing OptiLIGHT – a bright solution for dry eyes. OptiLIGHT elevates dry eye management with Lumenis’ patented Optimal Pulse Technology (OPT™) and user-centered design. Get the safe, precise, elegant procedure you want and the comfortable, effective therapy your patients need to manage dry eye disease with OptiLIGHT, a dry eye medical device.
THE FIRST AND ONLY IPL FDA APPROVED FOR DRY EYE MANAGEMENT
STOP THE DRY EYE
VICIOUS CYCLE OF INFLAMMATION
Reduce Inflammatory Mediators
Decrease the level of pro-inflammatory mediators to inhibit inflammation.1,2
Improve Tear Breakup Time
Significantly boost tear break up time and decrease osmolarity.6,7
Alleviate Abnormal Blood Vessels
Destroy the abnormal blood vessels that perpetuate inflammation.3,4
Restore Meibomian Glands
Improve meibomian gland morphology and functionality.2
Decrease Demodex
Decrease the population of Demodex mites, which stimulate infection and boost the bacterial load on eyelids.5
ELEVATING DRY EYE MANAGMENT
Every element designed to provide a precise and comfortable Dry Eye procedure

Patented OPT™ Handpiece
Each of your patients has individual, delicate facial contours. With the patented OPT™ handpiece, the treatment is easily customized to fit every curve and outline.
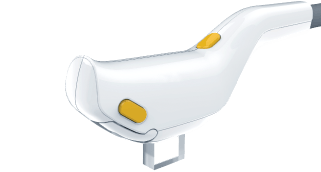
Ergonomic IPL Handpiece
Treat wider areas safely and comfortably using the IPL handpiece with patented SapphireCool™ technology to effectively break dry eye vicious cycle of inflammation.
Proven Settings And Protocols
Utilize embedded settings based on Lumenis’ clinically validated treatment protocols for safe and effective dry eye treatment.
Tailored Experience For Maximum Safety And Comfort
Experience optimal energy control with the Opti -Tip, which optimizes light energy for delicate areas, ensuring a safe, hygienic and effective treatment.
Optimal Pulse Technology (OPT™) That Transforms Light-Based Therapy
Get targeted, precise and controlled treatment with OptiLIGHT’s patented OPT™ technology – optimal energy with no-spike consistency.
ELEVATING YOUR DRY EYE PRACTICE
Light-based, quick, 15-Minute Procedure
Each OptiLIGHT session takes about 15 minutes, so you can easily integrate OptiLIGHT into your practice. With a seamless workflow and just 4 easy-to-perform sessions, OptiLIGHT is an essential tool in your dry eye toolkit. Elevate clinical effectiveness, practice efficiency and business growth with OptiLIGHT, dry eye medical device.
Visible results that last
Courtesy of Laura M. Periman, MD
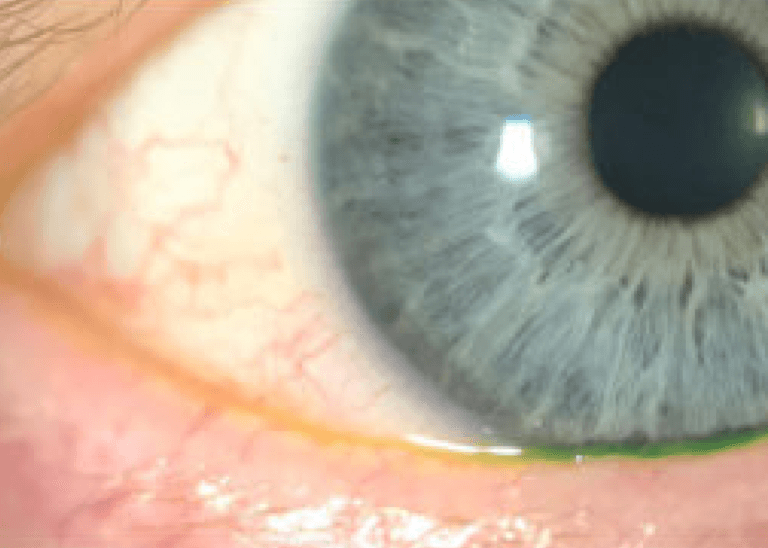
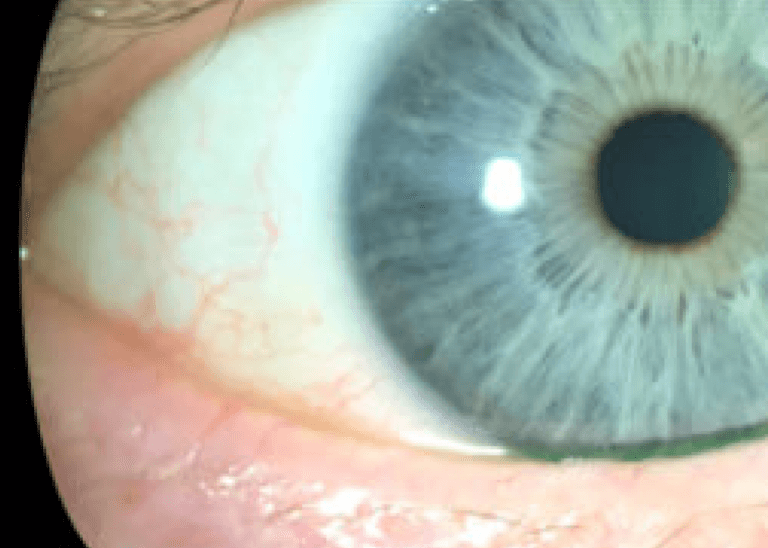
Visible results that last
Courtesy of Laura M. Periman, MD
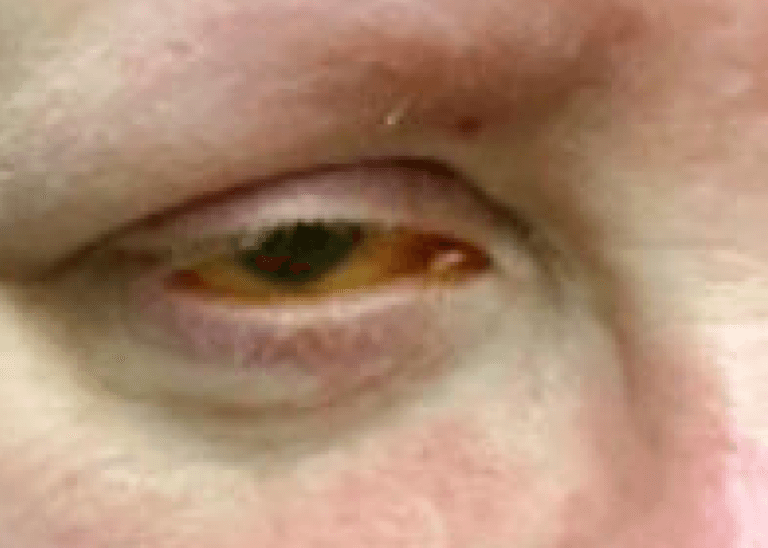
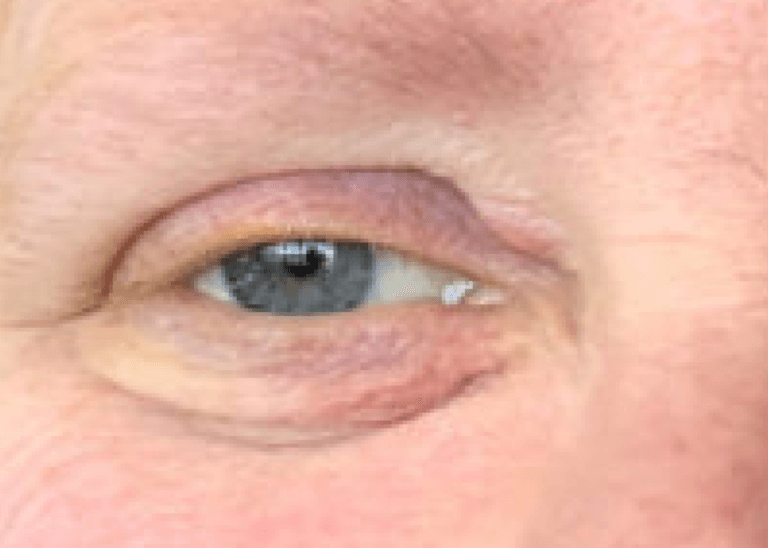
LUMENIS’ BRIGHT SOLUTION:
BACKED BY PEER-REVIEWED CLINICAL STUDIES*
Effective multifactorial treatment for a multifactorial disease
Decrease inflammation
Tear film level of IL-17 inflammatory marker is significantly reduced.1
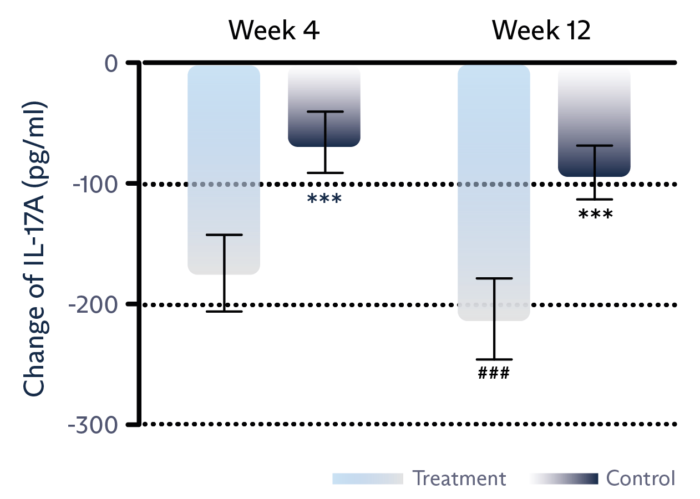
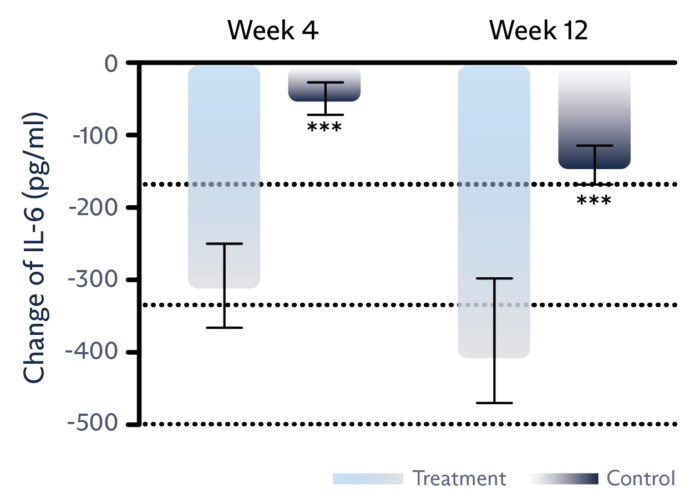
Decrease inflammation
Tear film level of IL-6 inflammatory marker is significantly reduced.1
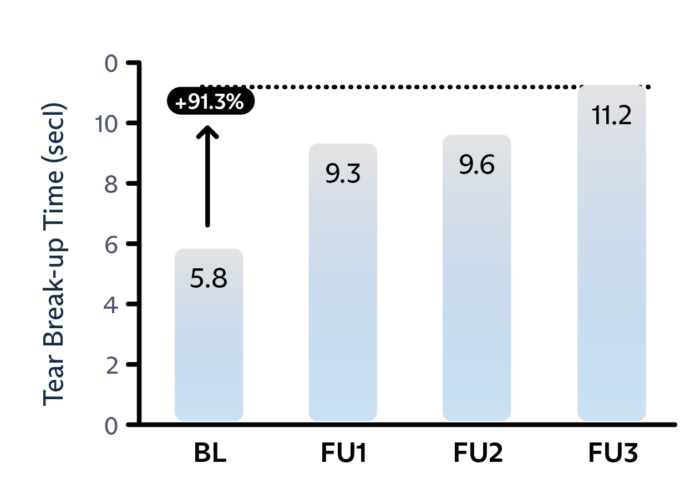
IMPROVE TEAR BREAKUP TIME
Treatment nearly doubles the tear breakup time.6

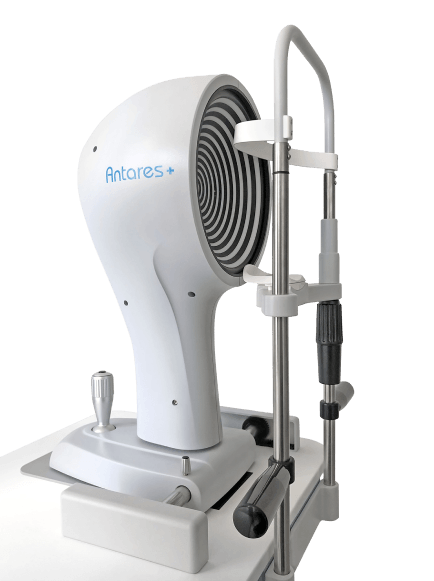
ANTARES DRY EYE DISEASE DIAGNOSTICS
Advanced, automated DED diagnostic and corneal topographer
Quality
Accurate, reliable, reproducible and low intra-rater variability
Speed
Fast examination process and results
Comfortable
Offers patient comfort as well as physician ease of use
Applications
Dry eye report, Meibography, Tear Breakup Time, Tear Meniscus, Topographer
LEARN HOW YOU CAN ELEVATE YOUR PRACTICE
Learn about the powerful pairing of OptiLIGHT’s patented OPT™ technology and OptiPLUS’ innovative dual frequency RF technology.

Download OptiLIGHT & OptiPLUS info kit
FAQ
OptiLIGHT is a light-based treatment that uses precise, intense broad spectrum light to address signs of dry eye disease due to meibomian gland dysfunction (MGD). MGD is the most common cause of dry eye disease, accounting for about 86% of cases8. The treatment addresses inflammation, which is one of the key underlying factors in MGD. OptiLIGHT was specifically developed to reach the delicate contours of the treated area safely, effectively, and gently using the OPT™ technology. It is intended for use in patients who are at least 22 years old, together with other available treatments, such as Meibomian gland expression, artificial tear lubricants, and warm compresses.
Lumenis light therapy is the first and only IPL FDA approved for Dry Eye management due to Meibomian Glands Dysfunction (MGD).
- Patented OPTTM handpiece
- Ergonomic IPL handpiece
- Personalized experience for safety and comfort
- Proven setting and protocols
- Transforming the use of light with OPTTM technology
Each treatment takes about 15 minutes, so you can easily integrate OptiLIGHT into the workflow. Patients achieve results in just 4 comfortable OptiLIGHT treatments.
Immediately following treatment, patients may experience some redness. This will usually disappear within a few hours.
Patients can resume their daily activities according to their doctor’s recommendation.
Lumenis’ patented technology OPT™ (Optimal Pulse Technology) transforms the use of light and allows targeted, uniform, precise, and controlled treatment. Specifically designed for the delicate area below the eye, OPT™ safely and effectively breaks the vicious cycle of inflammation associated with dry eye.
The wavelength range of IPL is between 400 and 1200 nm. The OptiLIGHT system limited the wavelength range to of 590-1200 nm obtained with the use of a cut-off filter of 590 nm.
OptiLIGHT Specifications
ExpertFilters*
OptiLIGHT; Acne: (400-600 & 800-1200); Vascular (530-650 & 900-1200); 515 nm, 560 nm; 590 nm; 615 nm; 640 nm; 695 nm; 755nm;
Lightguides
15x35mm, 8x15mm, 6.4mm Ø
Maximal Fluence**
35 J/cm2
Cooling
Continuous contact cooling for IPL handpiece
* OptiLIGHT filter is received complementary with the system. Other filters can be purchased separately
** For upgraded IPL aesthetic configuration
1. Liu et al. (2017), Am J Ophthalmol 183-190; 2. Yin et al. (2018), Curr Eye Res 43(3):308-313; 3. Kassir et al. (2011), J Cosmet Laser Ther 13(5):216-322; 4. Papageorgiou et al. (2008), Br J Dermatol 159(3):628-632; 5. Prieto et al. (2002), Lasers Surg Med 30(2):82-85; 6. Dell et al. (2017) Clin Ophthalmol 11:817-827; 7. Toyos & Briscoe (2016), J Clin Exp Ophthamol 7:6. 8. Lemp, et al. Cornea 31.5 (2012): 472-478
* All clinical studies cited here were conducted with Lumenis IPL with OPT technology.
OptiLIGHT is intended to be used by licensed practitioners, according to local rules and regulations.
Risks and warning (non-inclusive list):
In EU: Evaporative Dry Eye Disease (DED), also known as dry eye syndrome or lipid tear deficiency, due to Meibomian Gland Dysfunction (MGD). This indication is intended for Fitzpatrick skin types I-V.
In US: Improvement of signs of Dry Eye Disease (DED) due to Meibomian Gland Dysfunction (MGD), also known as evaporative dry eye or lipid deficiency dry eye, in patients 22 years of age and older with moderate to severe signs and symptoms of DED due to MGD and with Fitzpatrick skin types I-IV. IPL is to be applied only to skin on the malar region of the face, from tragus to tragus including the nose (eyes should be fully covered by protective eyewear). IPL is intended to be applied as an adjunct to other modalities, such as meibomian gland expression, artificial tear lubricants and warm compresses. The indications are only relevant where they were approved by the Regulatory Authorities.
Treatment with OptiLIGHT is contraindicated for patients with the following conditions in the treatment area: Ocular surgery or eyelid surgery or Neuro-paralysis within 6 months prior to the first treatment; Uncontrolled eye disorders affecting the ocular surface; Pre-cancerous lesions, skin cancer or pigmented lesions; Uncontrolled infections or uncontrolled immunosuppressive diseases; Recent Ocular infections; History of cold sores or rashes in the perioral area, including: Herpes simplex 1 & 2, Systemic Lupus erythematosus and porphyria; Use of photosensitive medication and/or herbs that may cause sensitivity within 3 months prior to the first IPL session; Recent radiation therapy to the head or neck or planned radiation therapy; Recent treatment with chemotherapeutic agent or planned chemotherapy; History of migraines, seizures or epilepsy. Patients eyes must be completely occluded during the treatment. Please refer to the operator manual for a complete list of intended use, contraindications and risks.
The following possible side effects can occur following IPL treatments: Pain/discomfort, damage to natural skin texture, change of pigmentation, scarring, excessive edema, fragile skin, bruising, burns, pruritus and xerosis. Please refer to the user manual or ask your doctor for a complete list of intended use, contraindications and risks.
PB-00030050 Rev C