Bosley and Lumenis Partner to Introduce Breakthrough Hair Loss Solution, FoLix™, to the U.S.

Lumenis’ first-in-class, proprietary and FDA-cleared fractional laser technology for hair loss treatment has the power to transform the lives of millions of hair loss sufferers in the U.S.
SAN JOSE, Calif., Sept. 24, 2024 /PRNewswire/ — Lumenis Be. Ltd., a leading energy-based medical device company for aesthetic and eye care solutions, announced a new partnership with Bosley, the largest hair restoration practice in the United States and a recognized leader in the industry. Bosley becomes the first national hair restoration medical practice in the United States to offer FoLix™, the first and only FDA-cleared fractional laser for hair loss treatment.

Recently cleared by the FDA, FoLix becomes the first and only fractional laser for safe, effective, and natural hair loss treatment for women and men in the United States.
Bosley will initially offer FoLix at its Beverly Hills location beginning September 2024 and plans to roll out the service at its other locations across the country over time. This partnership initiates a new era in hair loss treatment by introducing the first FDA-cleared fractional laser solution for hair loss to U.S. customers for the first time, potentially benefiting more than 80 million people affected by hair loss across the country1. The partnership also highlights Bosley’s dedication to supporting customers in their fight against hair loss, reinforcing its position as one of the world’s most trusted and experienced hair restoration experts.
“Staying at the forefront of innovations and excellence, we are always looking for the latest and most effective advancements to support our customers in their hair restoration journey,” said Rob Spurrell, CEO and President of Bosley. “FoLix is a strong and perfect fit for our expanding range of solutions, as it offers a non-surgical, in-office approach with minimal discomfort, showing promising results in several monthly sessions.”
“At Bosley, we understand the profound impact that hair loss can have on our patients’ lives. We have been helping patients with hair loss, surgically and non-surgically, with emerging technology for 50 years, and we are excited to introduce the Lumenis FoLix treatment, an innovative treatment designed to help our patients restore their hair and confidence,” said Ken Washenik, MD PhD, Chief Medical Officer at Bosley.
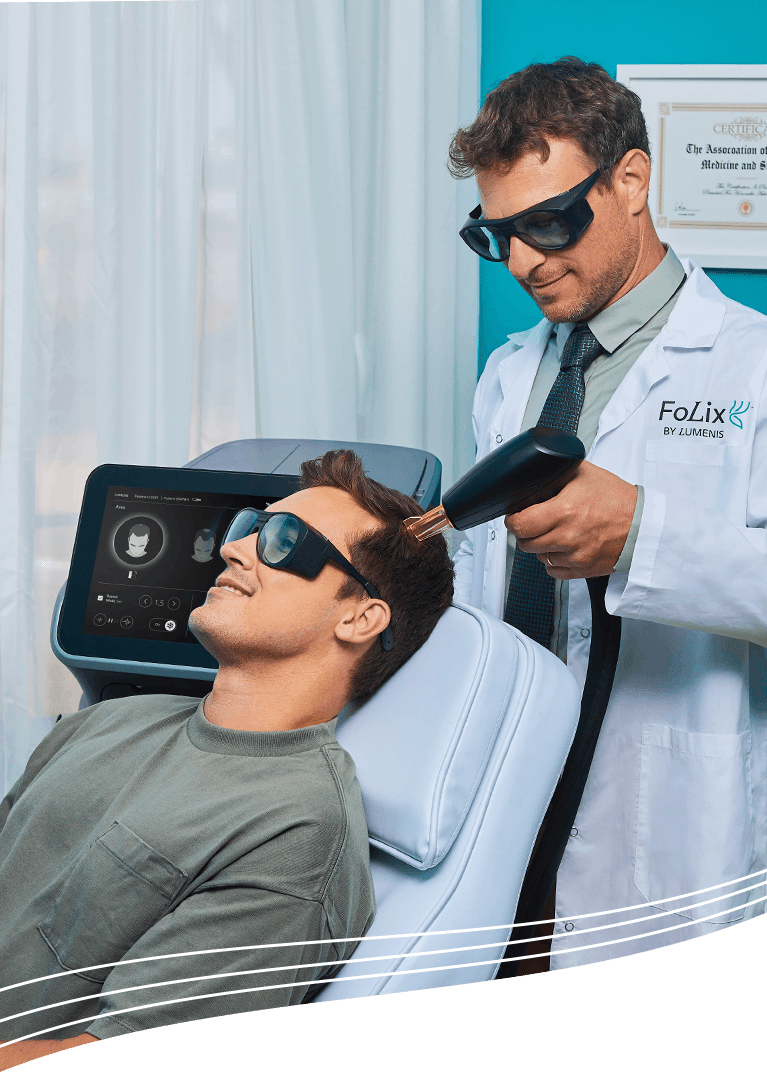
Following the FDA clearance granted earlier this year, Lumenis launched FoLix, a groundbreaking innovation in hair loss treatment and the first of its kind. Developed with Lumenis’ proprietary technology, FoLix offers a non-invasive, effective, simple, and safe solution that uses a unique approach to deliver precise pulses of laser light that leverage the body’s natural processes to stimulate hair follicles. FoLix is anticipated to attract considerable interest from people impacted by hair loss, as it offers results through 4-6 monthly sessions with no chemicals, needles, anesthesia, or downtime.
Lumenis and Bosley are both committed to raising awareness and educating about the causes, prevention, and treatment of hair loss. Hair loss is a common condition that could appear in various stages of life, with 2 in 3 men facing hair loss by age 352 and 1 in 2 women facing hair loss by age 503. The introduction of FoLix meets this strong clinical need and can have a significant impact on people’s self-esteem, confidence, and quality of life.
“With proven results from Lumenis’ pre-clinical and clinical studies, FoLix represents a significant leap forward in the world of hair restoration for both patients and providers,” said Brad Oliver, President of Lumenis Americas. “Our partnership with Bosley is an exciting first step in bringing FoLix to the U.S. market, and we are confident that this revolutionary technology will make a meaningful difference in the lives of millions of people who suffer from hair loss, empowering them to take control and grow more confident in their everyday experiences.”
For more information about FoLix™, please visit Lumenis.com/FoLix
About Lumenis
Lumenis is a global leader in the medical aesthetic market and is a world-renowned expert in developing and commercializing innovative energy-based technologies, including Laser, Intense Pulsed Light (IPL) and Radiofrequency (RF). For more than 50 years, Lumenis’ ground-breaking products have redefined medical treatments and set technological and clinical gold-standards, revolutionizing existing treatment methods, and creating solutions for previously untreatable conditions. Lumenis is a portfolio company of EQT Private Capital Asia. For more information regarding Lumenis’ range of clinical solutions, please visit: www.lumenis.com.
About Bosley
Founded in 1974, Bosley is the largest hair restoration practice in the United States. For more than 50 years, Bosley physicians have focused on the art and science of hair restoration for both men and women, utilizing innovative, artistic and scientific techniques. With more than 500,000 hair restoration procedures performed (with patients from all 50 states and 60 other countries), Bosley has helped hundreds of thousands of patients find a permanent solution for their hair loss. For more information, please visit www.bosley.com.
About FoLix™
FoLix™ is a non-ablative fractional laser device indicated for improving the appearance of scalp hair in adult males and females with Fitzpatrick skin types I to IV, who are seeking treatment for hair loss.
FoLix™ treatment could cause redness, swelling, scarring, and pigmentation change. The use of FoLix is contraindicated for patients with any concurrent cancer or history of skin cancer, active infection, fungal or bacterial diseases. See the system user manual for a complete list of contraindications and risks.
Media Contact
Andrew Spivak, andrews@bosley.com.
Courtney Myers, courtney.myers@havasred.com.
- According to the American Academy of Dermatology Association.
- According to the American Academy of Dermatology Association.
- According to MedlinePlus by NIH.
DOWNLOAD FoLix BROCHURE
PB-00081710 Rev A