FoLix™ by Lumenis Wins Innovation Award in Hair Loss Technology at the 2025 Medical Device Network Excellence Awards

Lumenis is thrilled to announce that FoLix™, our groundbreaking hair loss treatment device, has been named a winner in the 2025 Medical Device Network Excellence Awards, receiving recognition with the Hair Loss Technology Innovation Award. The Medical Device Network Excellence Awards honor the most significant achievements and innovations in the medical devices industry. Powered by GlobalData’s business intelligence, the Awards recognize the people and companies leading positive change and shaping the future of the industry.
“We understand that experiencing hair loss can have a profound impact on patients. We’re honored that FoLix, the first FDA-cleared non-ablative fractional laser for hair loss, has been recognized as the premier treatment choice for restoring confidence and helping people feel like their best selves.”
— Tzipi Ozer-Armon, CEO of Lumenis
The New Standard in Hair Loss Treatment
Hair loss affects more than 80 million people in the U.S., with significant emotional and psychological consequences. Despite its prevalence, many existing treatments fall short—whether due to limited efficacy, side effects, or the invasiveness of surgical procedures.
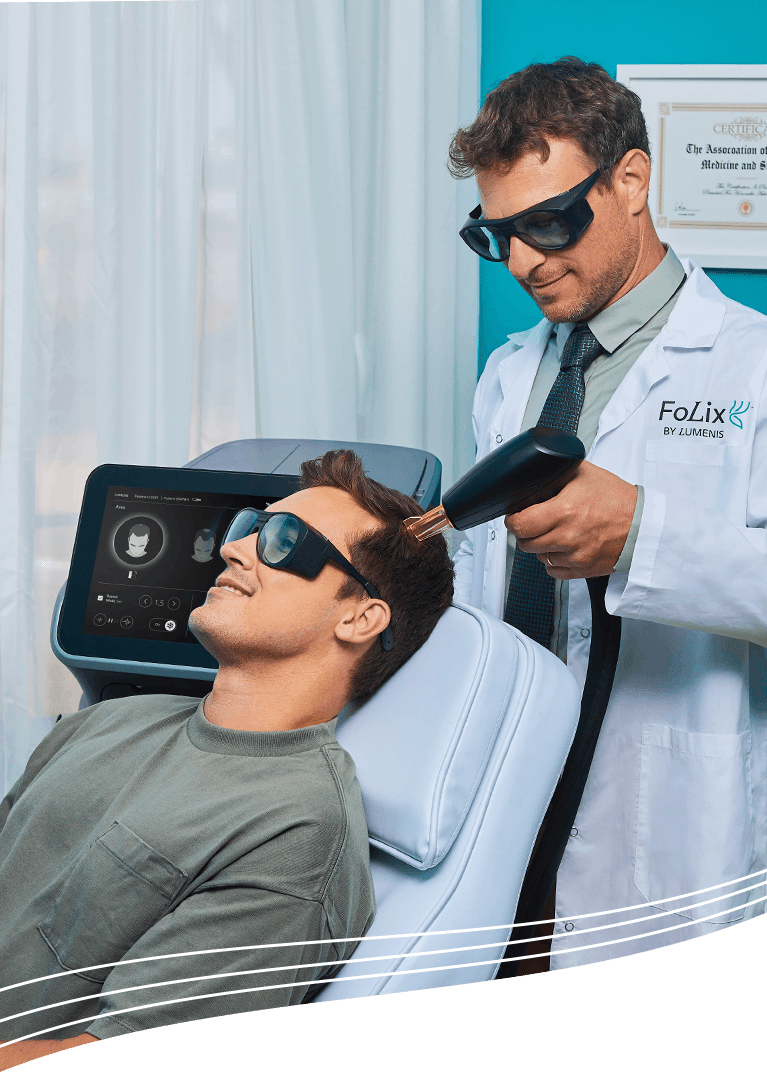
FoLix offers a non-invasive, clinically validated alternative that is safe, effective, and accessible to both men and women. As the first and only FDA-cleared non-ablative fractional laser (NAFL) for hair loss, FoLix uses Lumenis’ proprietary NAFL technology to stimulate hair follicles by activating the body’s natural healing processes. The treatment protocol requires just four to six monthly sessions, plus maintenance 2-3 times a year to sustain and enhance the results. Each session is quick and comfortable, with no downtime.
This innovation is underpinned by a unique mechanism of action: the creation of microscopic thermal zones (MTZs) in the skin, which triggers a regenerative response without damaging surrounding tissue. The technology’s specificity and safety profile are further validated by its FDA clearance, a milestone that sets a new industry standard for device-based hair loss treatment. By leveraging decades of expertise in energy-based medical devices, Lumenis has established a new category of treatment that is both effective and accessible.
Clinically Proven, Patient-Centric Innovation
FoLix’s effectiveness is backed by rigorous clinical validation*. Preclinical studies have demonstrated that NAFL treatment induces a natural inflammatory response that stimulates hair follicle transition into a growth state. For example, in controlled studies on mice, NAFL treatment led to significant overexpression of key hair growth markers such as Sonic Hedgehog (SHH) and activation of the Wnt/B-catenin pathway, both of which are critical for hair follicle development and regeneration. Histological analysis confirmed positive tissue repair and hair follicle regeneration post-inflammatory response.
In clinical settings, retrospective studies involving human subjects have reinforced these findings. In a review of 98 patients aged 21-66, who underwent three to six FoLix treatments, independent board-certified dermatologists were able to correctly identify before-and-after images in 96.9% of cases. Both male and female subgroups demonstrated high rates of improvement, with no adverse events reported during or after treatment. Prospective studies further provided quantitative results that demonstrate an increase in hair count per square centimeter and an increase in the number of follicular units per square centimeter. The high safety profile and the statistically significant improvements in scalp hair appearance underscore the reliability of the technology for a broad patient population.
Driving Global Impact Through Strategic Partnerships
FoLix’s rapid growth has been fueled by partnerships with leading dermatologists and hair restoration experts, including the NYU Dermatology Department, Bauman Medical, and Dr. Ronda Farah. Lumenis’ collaboration with Bosley, the largest hair restoration chain in the U.S., has accelerated clinical adoption and patient access. Within its first year, FoLix established a presence across North America, the Middle East, and the Asia-Pacific region, with continued global expansion driven by strong demand from physicians and patients worldwide**.
Looking Ahead
As we continue to invest in digital health, remote connectivity, and smart device ecosystems, Lumenis is shaping the future of energy-based medical devices. Our vision includes real-time adaptive treatments, remote troubleshooting, and personalized care that enhances both clinical outcomes and operational efficiency.
FoLix is just the beginning. We’re exploring new technologies and indications to further expand our impact—driven by innovation, validated by science, and inspired by the patients and providers we serve.
DOWNLOAD FoLix BROCHURE
* Avram, M.R., Queen, D., Shapiro, J. and Munavalli, G. (2025), Improvement in Scalp Hair Appearance Following Treatment With a Non-Ablative Fractional Laser: A Retrospective Observational Study. Lasers in Surgery and Medicine, 57: 590-597. https://doi.org/10.1002/lsm.70044.
** FoLix availability varies by region and is subject to local regulatory approvals in each jurisdiction.
PB-00095090 Rev A