Lumenis introduces FoLix™, the first FDA-cleared proprietary fractional laser for hair loss treatment

June 5, 2024 – Yokne'am Illit, Israel
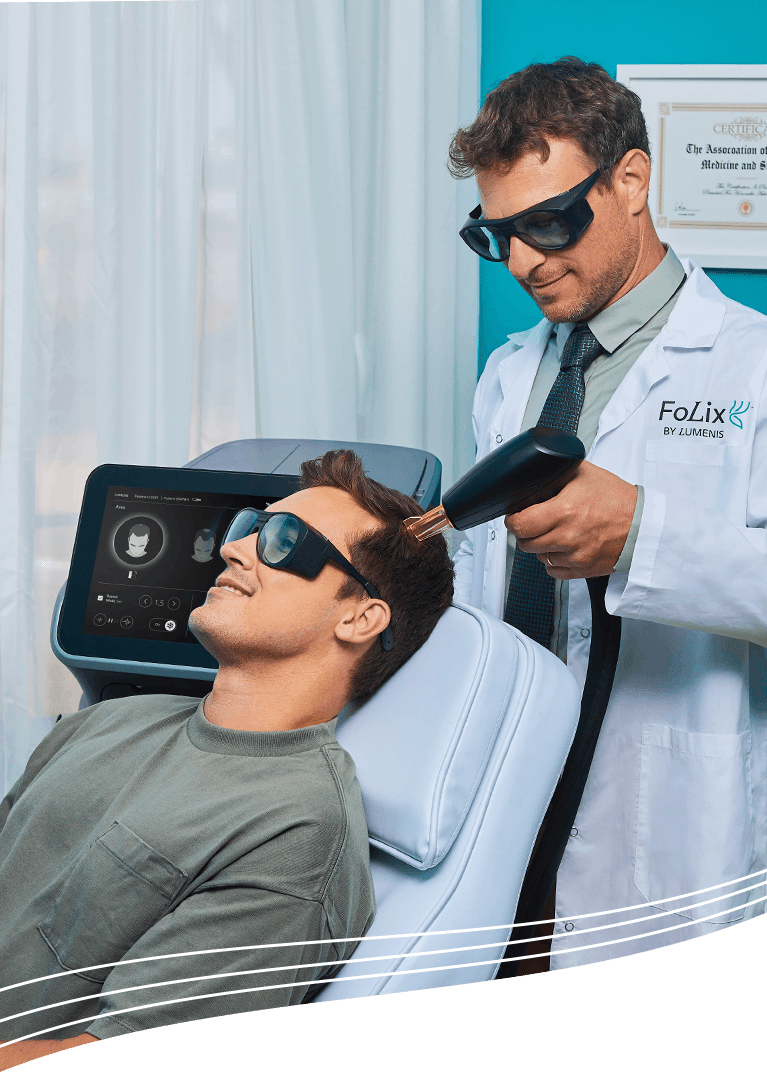
Lumenis Be. Ltd., a leading energy-based medical device company for aesthetic and eye care solutions, is proud to announce the launch of its breakthrough laser system designed to combat hair loss. Recently cleared by the Food and Drug Administration (FDA), FoLix becomes the first and only fractional laser choice for safe, effective, and natural hair loss treatment for women and men in the United States1.

Affecting more than 85% of men and 50% of women2, hair loss remains a persistent, widespread challenge. FoLix’s groundbreaking approach utilizes fractional laser and Lumenis’ proprietary technology tailored for hair. This is a revolution for hair loss patients, offering results in 4-6 monthly sessions with no chemicals, needles, anesthesia, surgery, or downtime.
FoLix’s unique technology delivers precise pulses of laser that leverage the body’s natural processes to stimulate hair follicles. Lumenis’ pre-clinical and clinical studies measuring the laser’s effect showed a positive impact on hair growth, with noticeable improvements in scalp hair appearance and an increase in hair count3. “Fractional lasers have been shown to have remarkable effects on the skin and now, finally, Lumenis has been able to harness that power and bring an innovative solution for hair stimulation.” Said Dr. Marc Avram, a renowned hair transplant specialist and Clinical Professor of Dermatology at Weill Cornell Medical College (WCMC).
“With hair loss becoming a growing concern, FoLix emerges as a pivotal innovation for both patients and providers” adds Dr. Neil Sadick, a world-leading dermatologist and researcher, and Clinical Professor of Dermatology at WCMC. “FoLix offers a unique opportunity to help those suffering from hair loss through short monthly sessions and with minimal discomfort and no downtime.”
“At Lumenis, we’re committed to transforming lives through science and innovation. FoLix is about more than just hair; it’s about having the self-confidence to live life to the fullest,” said Lumenis CEO Tzipi Ozer-Armon. “We are eager to collaborate with providers across the US to extend the influence and accessibility of this groundbreaking solution.”
For more information about FoLix please visit Lumenis.com/FoLix.
About Lumenis
Lumenis is a global leader in the medical aesthetic market and is a world-renowned expert in developing and commercializing innovative energy-based technologies, including Laser, Intense Pulsed Light (IPL) and Radiofrequency (RF). For more than 50 years, Lumenis’ ground-breaking products have redefined medical treatments and set technological and clinical gold-standards, revolutionizing existing treatment methods, and creating solutions for previously untreatable conditions. Lumenis is a portfolio company of EQT Private Capital Asia. For more information regarding Lumenis’ range of clinical solutions, please visit: www.lumenis.com
About FoLix
FoLix is a non-ablative fractional laser device to improve the appearance of scalp hair in adult males and females with Fitzpatrick skin types I to IV, who are seeking treatment for hair loss.
FoLix treatment could cause redness, swelling, scarring, and pigmentation change. The use of FoLix is contraindicated for patients with any concurrent cancer or history of skin cancer, active infection, fungal or bacterial diseases. See the system user manual for a complete list of contraindications and risks.
*The physicians’ testimonials represent a specific experience of a paid spokesperson. Experience of others may vary.
1 See the “About FoLix” section for the indication of use and relevant skin types.
2 Men’s Hair Loss. American Hair Loss Association- https://www.americanhairloss.org/mens-hair-loss/ and Hair Loss in Women. Cleveland Clinic- https://my.clevelandclinic.org/health/diseases/16921-hair-loss-in-women
3 As measured in 1-month and 3-month follow-ups following the last treatment session, compared to baseline. Data on file.
DOWNLOAD FoLix BROCHURE
PB-00079380, Rev A