LASER HAIR LOSS TREATMENT
Hair Loss is More Common Than You Think.
Hair thinning is a concern shared by millions, often prompting individuals to seek expert solutions. Offering effective guidance starts with understanding the underlying causes and the full range of treatment pathways. Integrating trusted solutions into your offering empowers you to address patient needs with confidence and elevate the level of care you provide.


HAIR LOSS IN NUMBERS
80M
Americans are effected by Hair loss (50M men & 30M women).
50% OF WOMEN
of women experience hair loss by the age of 50.
85% OF MEN
Will experience hair loss in their lifetime.
87%
Agree that the condition of their hair greatly influences their confidence.

WHAT IS HAIR LOSS?
Experiencing hair loss is more common than you think. More than 50% of Americans experience hair loss at some point in their lives. The causes of hair loss can vary, including genetics, hormonal changes, stress, certain medications, and aging.
There are several different types of hair loss:
Androgenetic Alopecia (AGA)
Natural, gradual, common hair loss as a result of changes in androgen levels that usually come with age.
Alopecia Areata (AA)
Autoimmune disease where the immune system mistakenly attacks the hair follicles.
Telogen Effluvium
A form of hair loss that is triggered by physical trauma, psychological stress or other types of unexpected change.


Courtesy of LaserMed clinic
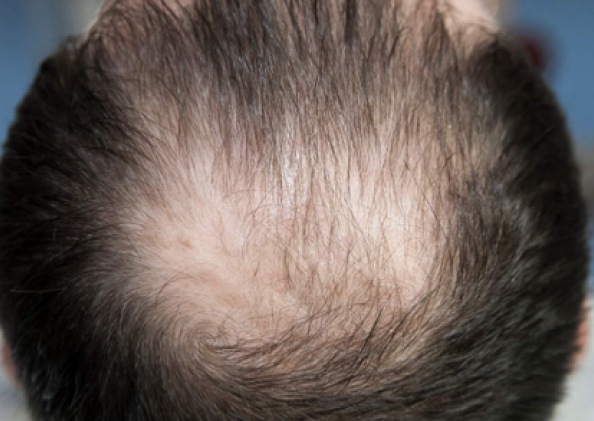
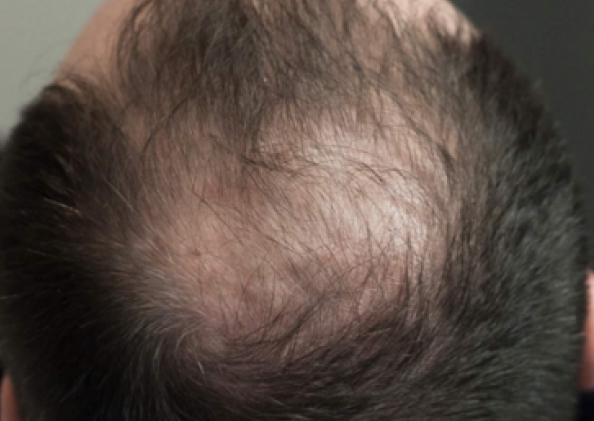
Courtesy of LaserMed clinic
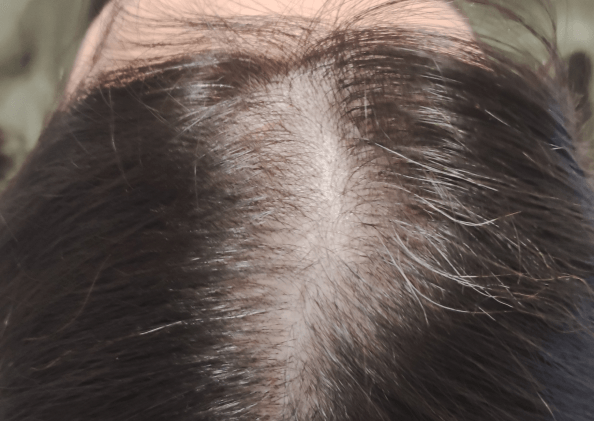
Courtesy of LaserMed clinic
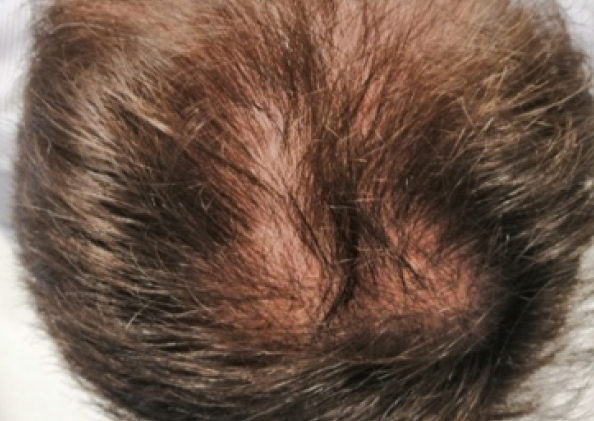

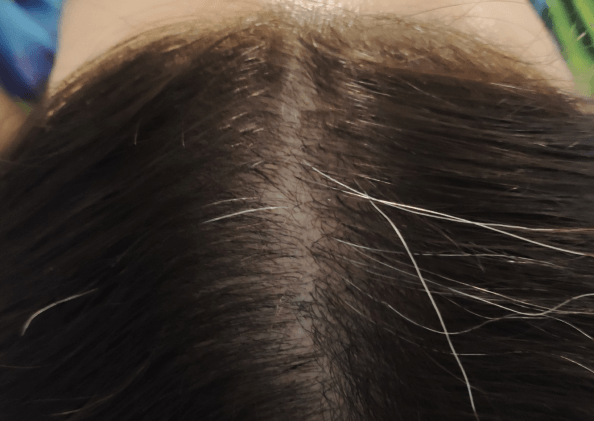
Courtesy of LaserMed clinic

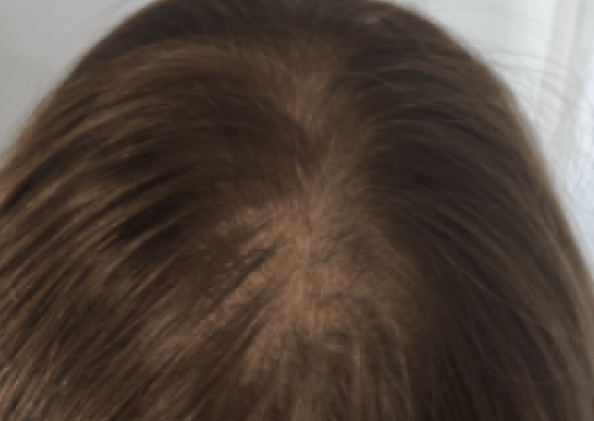
Courtesy of LaserMed clinic
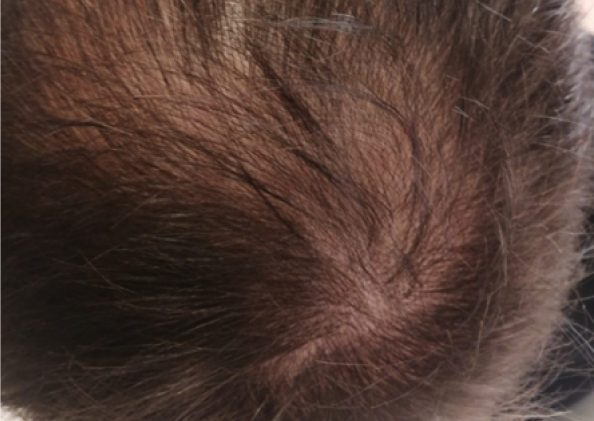

Courtesy of LaserMed clinic
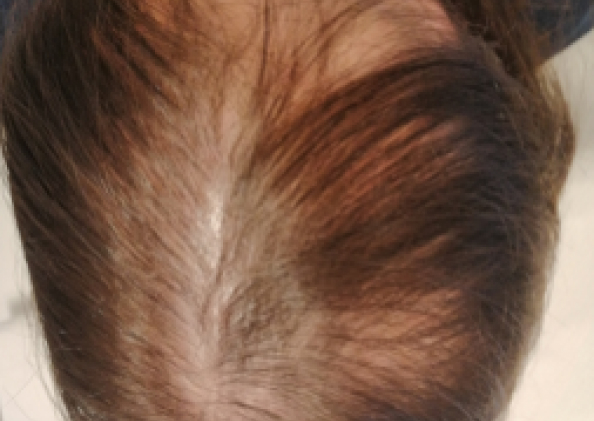

Courtesy of LaserMed clinic
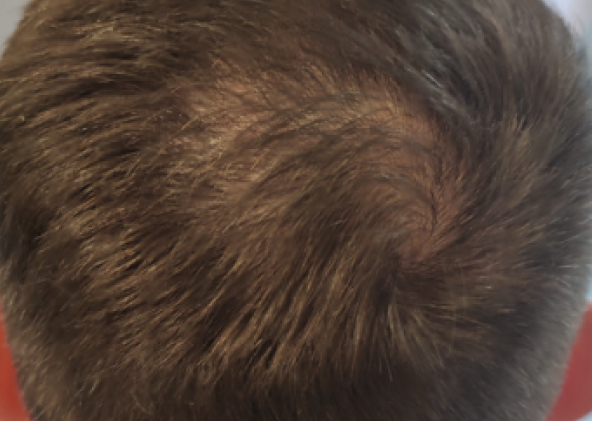

Courtesy of LaserMed clinic
THE HAIR GROWTH CYCLE
Throughout life, hair follicles undergo cyclic degeneration and regeneration-between 10 and up to 30 cycles for each hair follicle.
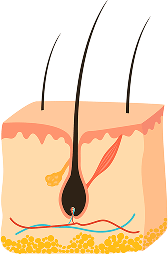
STAGE 1
ANAGEN (GROWTH)
The longest phase, lasting 2-8 years, where the follicle actively produces the hair shaft. Hair length potential is determined by this phase’s duration, which naturally shortens with age.
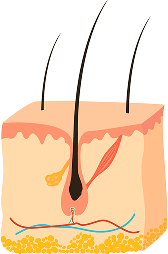
STAGE 2
CATAGEN (TRANSITION)
A brief 2-week phase where the follicle detaches from the dermal papilla and regresses. The dermal papilla must reach the follicle bulge during this time, or the hair will be shed.
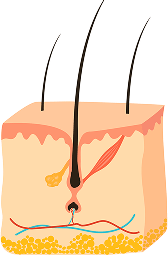
STAGE 3
TELOGEN (RESTING)
A 2-3 month period where growth stops and new hair begins forming at the follicle base. Typically, 10-15% of scalp hairs are in this phase at any time.
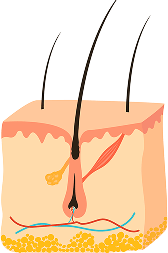
STAGE 4
EXOGEN (SHEDDING)
The final phase where hair is naturally shed unless the follicle re-enters the anagen phase. Normal daily hair loss ranges from 50-100 strands.
THE LUMENIS SOLUTION
FOR HAIR LOSS
FoLix represents a groundbreaking advancement in laser hair loss treatments. This revolutionary system leverages proprietary fractional laser technology to naturally stimulate hair follicles through enhanced blood flow and growth factor activation. The new FDA-cleared treatment for hair loss, FoLix offers a safe, non-invasive, chemical-free alternative to traditional hair loss solutions, delivering proven results through 4-6 monthly sessions.
FoLix Technology
FoLix uses fractional laser energy delivery for dermal coagulation, activating natural healing processes. FoLix uses laser technology to harness the body’s natural processes. The laser’s interaction with the tissue promotes enhanced blood circulation, cytokine activity, and stimulates hair follicles.
FAQ’s
FoLix is the first and only FDA-cleared non-ablative fractional laser for hair loss, providing effective, safe, simple and non-surgical hair loss treatment for women and men.
Each FoLix treatment session lasts approximately 30 minutes, making it easy to fit into a busy schedule.
Typically, patients need between 4 to 6 sessions, spaced about four weeks apart to achieve optimal results.
Patients generally report minimal discomfort during FoLix sessions, often describing the experience as a warm prickling sensation.
The hair growth cycle is long, so when it comes to treating hair loss, patience is key. Initial signs of improvement, such as reduced shedding and the appearance of baby hairs, can be noticed throughout the treatment series.
The optimized protocol consists of 4-6 sessions conducted every four weeks, followed by 2-3 yearly maintenance treatments to sustain and enhance results. Each treatment session takes approximately 30 minutes.
FoLix is indicated for adult males and females with Fitzpatrick skin types I to IV who are seeking treatment for hair loss. Early to mid-stages of hair loss are expected to show the utmost results. However, it is contraindicated for some diseases- see warning and risks for full information.
FoLix uses targeted laser energy for dermal coagulation, which activates natural healing processes. This mechanism enhances blood flow and cytokine activity, leading to hair follicle stimulation.
While FoLix is designed with patient safety in mind, the system incorporates FDA-cleared settings and the FoLiCool™ tip for enhanced safety and comfort. The intuitive user interface ensures consistent, efficient treatments. Potential side effects may include redness, swelling, scarring, burns and pigmentation changes – see warning and risks for full information.
FoLix availability varies by region and is subject to local regulatory approvals in each jurisdiction.
Indication for use: Improving the appearance of scalp hair in adult males and females with Fitzpatrick skin types I to IV, who are seeking treatment for hair loss.
Contraindication and Risks: The use of FoLix is not suitable for everyone, contraindicated for certain patients and carries some risks. FoLix treatment is not suitable for patients with any concurrent cancer or history of skin cancer, or pre-cancerous lesions at the treatment area, active infection, chronic fungal or bacterial diseases or chronic dermatological condition of the scalp. Treatment may cause redness, swelling, scarring, damage to natural skin texture (e.g. blisters), fragile skin, burns, hair shedding, itching, and pigmentation change. Be sure to consult with us before choosing this treatment.
*Measured in 1-month and 3-months follow ups following last treatment session, compared to baseline. Data on file.
**Avram, M.R., Queen, D., Shapiro, J. and Munavalli, G. (2025), Improvement in Scalp Hair Appearance Following Treatment With a Non-Ablative Fractional Laser: A Retrospective Observational Study. Lasers in Surgery and Medicine, 57: 590-597. https://doi.org/10.1002/lsm.70044.
A testimonial represents a specific experience of a paid spokesperson. Experience and results of others may vary.
The presenter in FoLix’s marketing materials isn’t a licensed practitioner. The footage is a demonstration, treatment must be done by licensed practitioners wearing protective eyewear.
References:
American Academy of Dermatology. Hair Loss Resource Center
Cleveland Clinic. Hair Loss
American Hair Loss Association
U.S. National Library of Medicine
Lumenis survey 2025, 802 patients experiencing hair loss (51% male, 48% female, and 0.3% other)
PB-00085300 Rev A