CLOSING THE GAP
IN YOUR PRACTICE
Tone The Muscles To Address Lower Lid Laxity And Impaired Blinking With The New OptiLIFT™ Device
LIFT UP YOUR PRACTICE WITH LUMENIS’ INNOVATIVE DEVICE
Address Lower Lid Laxity and Impaired Blinking
By strengthening the muscles in the periorbital area, OptiLIFT addresses the gravitational descent of the lower lids that can result in ineffective blinking
Proprietary Dynamic Muscle Stimulation Technology
Lumenis’ DMSt uses electrical pulses to trigger nerve signals, activating facial muscles and inducing contractions
Ideal Fit For
Eye Practice
OptiLIFT applicator is specifically designed for the periorbital area, featuring three predefined presets for treating patients’ delicate facial contours
Clinically
Validated
OptiLIFT is easy-to-use, safe and clinically validated to address blinking and lower lid laxity
Tone and Tighten the Periorbital Muscles
OptiLIFT stimulates the muscles with DMSt technology to tone and tighten the periorbital area, targeting the natural sagging that comes with aging
THE POWER OF
OPTILIFT TECHNOLOGY
Non-Surgical Device to Tone Muscles to Address Droopy Eyelids
Aging weakens periorbital muscles, often leading to eyelid laxity, reduced tone and impaired blinking. OptiLIFT’s Dynamic Muscle Stimulation (DMSt) activates muscle contractions, enhances tone, and addresses both the function and appearance of the lower eyelids.

TONE THE MUSCLES
BEFORE & AFTER
OptiLIFT delivers impressive and visible outcomes in just four easy sessions. It restores the tightness and tone of the muscle and enhances blinking efficiency, offering a gentle, non-invasive approach for a lifted appearance.
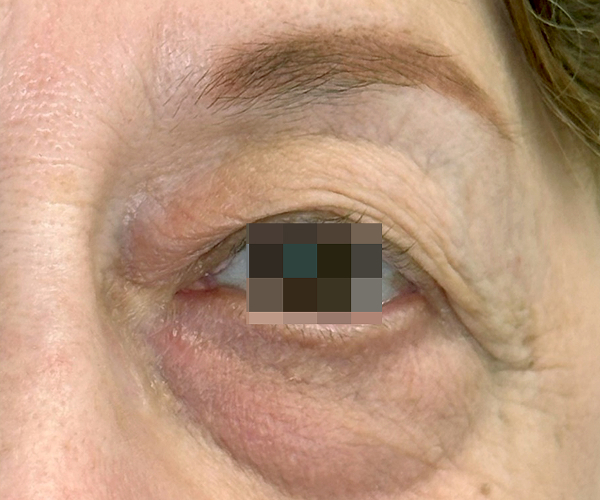
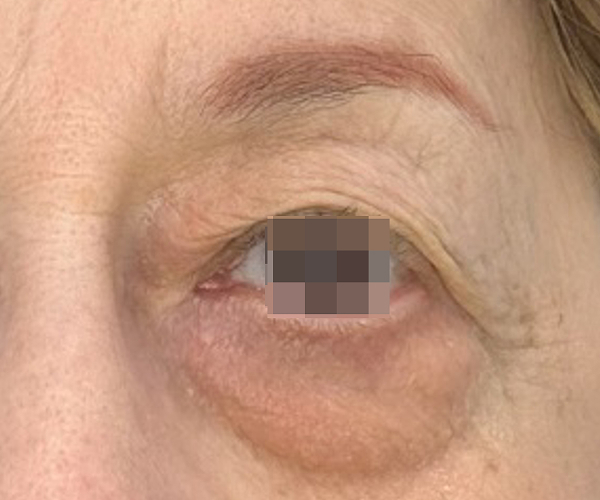
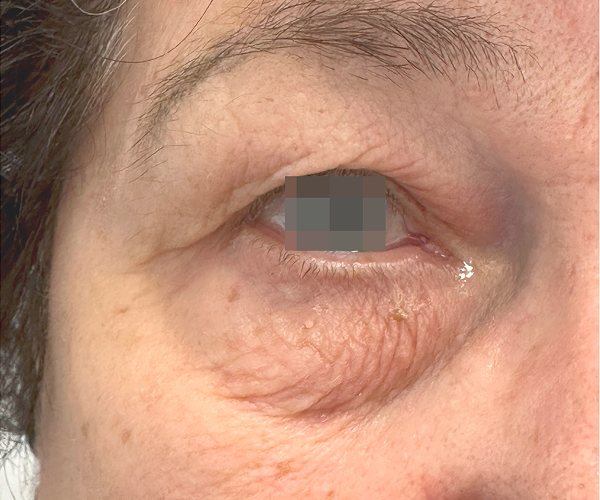
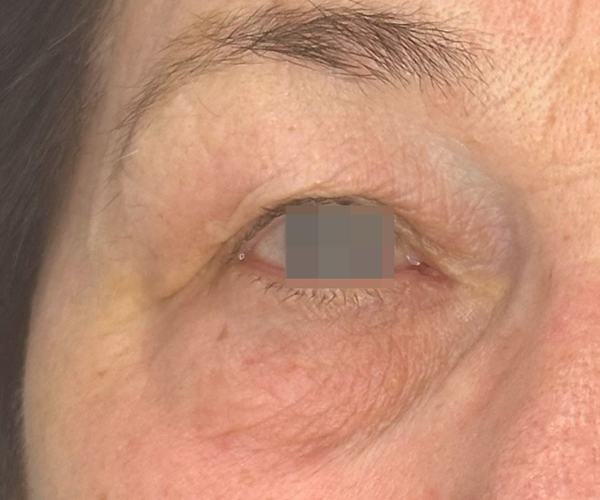
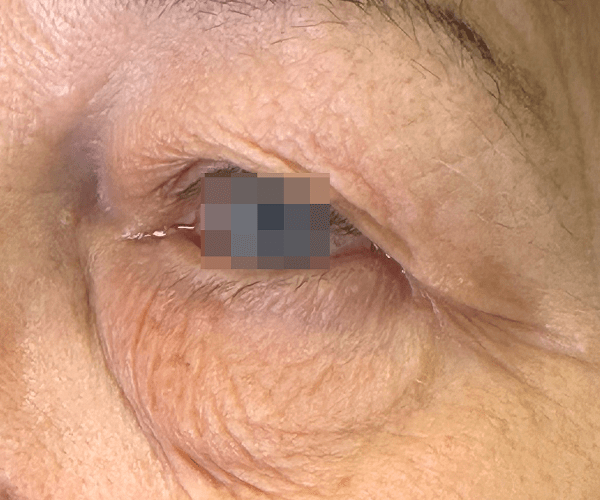
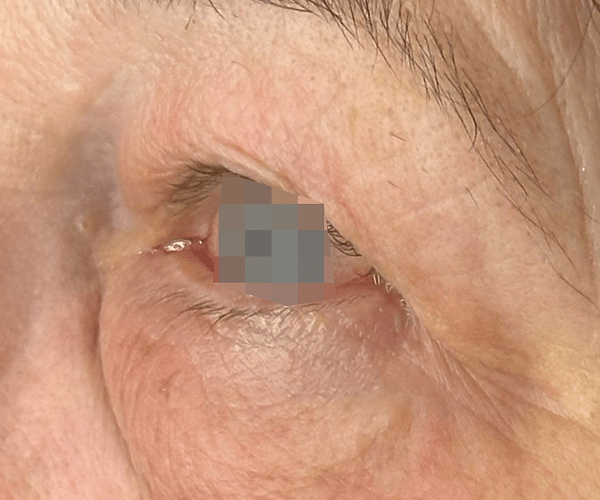
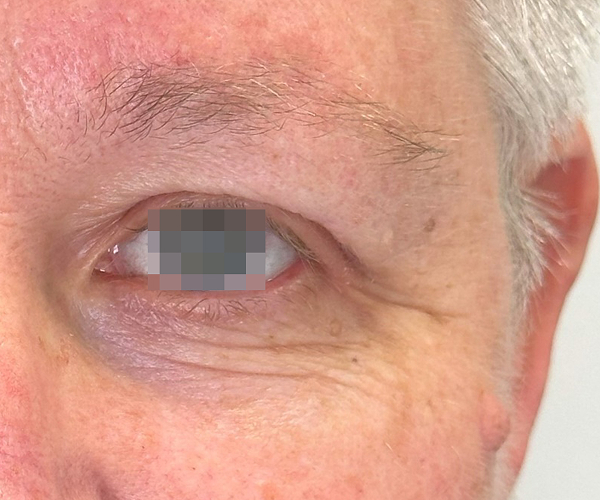
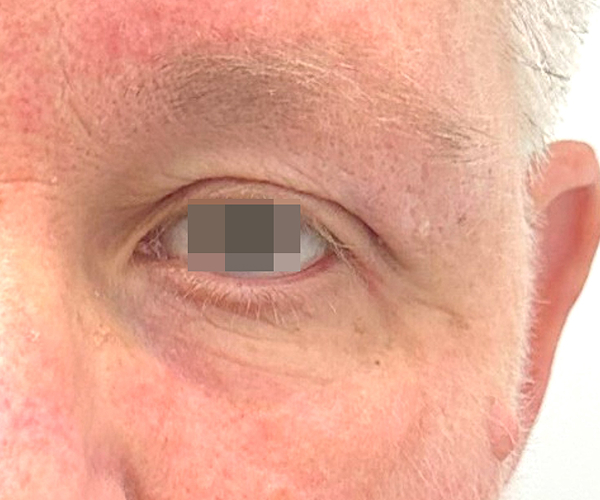
Courtesy of Dr. Dorina Cheles
CLINICALLY PROVEN TO ELEVATE YOUR PRACTICE
OptiLIFT is clinically validated1, with Dynamic Muscle Stimulation technology applied below the eyelids delivering results such as:
- 286% improvement in tear breakup time (TBUT)
- 70% Improvement in blinking rate and quality
- Decrease of at least 75% in lower lid laxity
- Enhanced meibomian gland function, improving signs of DED
WHAT DOCTORS
ARE SAYING
“With Lumenis’ Dynamic Muscle Stimulation technology, we can tone the muscles around the eye, to tighten the lower eyelid and create a lifted look, without surgery”

MD, FACS
Manhattan Face and Eye,
NewYork
“By strengthening the lower lid muscles with Lumenis’ DMSt and RF technologies, I can fight the natural sag that comes from aging”

MD
Periman Eye Institute
Seattle, Washington
“The missing link in addressing lower lid laxity. I’m able to keep patients in the practice and address their concerns without an invasive surgical approach”

OD, ABO
Lehigh Eye Sp cialists,
Allentown, Pennsylvania
“Having Lumenis’ DMSt technology in-office has been a game changer – I’ve seen positive results and steady revenue growth from medical and cosmetic treatments”

OD,
Everett Eye Care Center,
Everett, Pennsylvania
** The testimonials of the OptiLIFT believers and the providers represent specific experience of a paid spokesperson. The experience of other people may vary.
A PARTNERSHIP
YOU CAN TRUST
Experience the Lumenis Advantage & Build Your Future.
Partner with the pioneers who revolutionized dry eye care. Lumenis is your trusted partner for professional growth, clinical excellence, and expanding your practice—delivering innovative solutions that have been setting the gold standard in eye care for over 50 years.


DOWNLOAD INFO KIT
Discover how OptiLIFT can transform your practice.
Contact us for a free demo or to learn more!
1 Chelnis JG, Chelnis A. Dynamic Muscle Stimulation of the Periorbital Area for Improvement of Blinking in Dry Eye Patients. Clin Ophthalmol. 2025;19:1057-1071
Indication for use:
The OptiLIFT System and its accessories are intended for dermatological procedures requiring ablation and resurfacing of the skin when using VoluDerm Energy (Microneedling Applicator). It is also intended for use in dermatologic and general surgical procedures for non-invasive treatment when using RF Energy and for muscle conditioning to stimulate healthy muscles (OptiLIFT Applicator). OptiLIFT System is not intended to be used in conjunction with therapy or treatment of medical diseases or medical conditions of any kind. OptiLIFT System is intended to be operated by a licensed healthcare practitioner who is present to monitor treatment.
Warnings and risks:
OptiLIFT System is contraindicated for patients with pacemakers, defibrillators, any implanted electronic device, or metal implanted in the treatment area.
Side effects may appear either at the time of treatment or shortly after. Side effects may include any of the following: prolonged or significant pain, damage to natural skin texture (blister, burn), excessive skin redness (erythema), excessive swelling (edema), fragile skin bruising, excessive itching, change of pigmentation (hyper-pigmentation or hypopigmentation), scarring transient skin break-out such as acne and pimples. For a complete list of contraindications, please refer to the User Manual.
PB-00086860, Rev A